Monday’s Lesson: Quantum Chemistry at Your Fingertips
The interactions among electrons and nuclei are fundamentally important in determining the chemical properties of atoms and molecules. It is the gain, loss, and sharing of electrons that governs all chemical reactions. A chemical bond is created when two or more atoms share their electrons. The distribution of electrons in atoms and molecules affects how they interact and form various structures and states. Understanding the properties of electrons is a key to understanding chemistry.
Fundamental concepts in chemistry can be explained from the perspective of electronic interactions. In this lesson, which you will complete online, your students will be guided through a sequence of interactive simulations powered by a real-time computational quantum dynamics engine called the Quantum Workbench, embedded in our Molecular Workbench software.
But don’t be intimidated by the word “quantum.” The Quantum Workbench uses dynamic, interactive visualizations to teach the same concepts?—?like electron clouds and chemical bonding??—?you’ve seen in static textbook illustrations. For example, your students can drag the nucleus around to see how the electron cloud follows it. This explains why an atom that consists of a nucleus and an electron cloud can move as a whole.
Students can also:
- change the charge on the nucleus to see why the electron cloud cannot form an atom with a single neutron or why a negatively charged ion cannot take more electrons.
- apply an external electric field to see how an atom can be polarized or ionized, depending on the intensity of the field.
- add two nuclei and vary their electronegativities to see how they affect the formation of a covalent bond (see below).
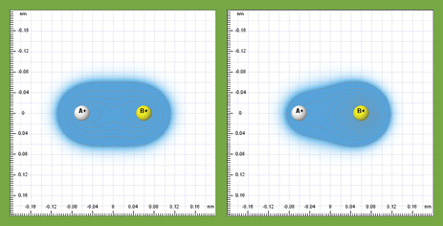
While these concepts are widely taught in chemistry, the Quantum Workbench provides a unique inquiry tool for students to learn about them in a profound way. All these complex, abstruse concepts can be reduced to a single, coherent picture: the electron cloud interacts with nuclei through electrostatic interaction and the electrons move around accordingly.
The Quantum Workbench has deep roots in science, which lends it unusual explanatory power. Quantum chemistry —?which explains chemistry using quantum mechanics?—?is an important milestone in the history of science, as witnessed by at least three Nobel Prizes awarded to its pioneers. The Quantum Workbench brings a small part of its power to your students’ fingertips and demonstrates its enormous educational potential.
Charles Xie (qxie@concord.org) is the creator of the Molecular Workbench software and the director of the Electron Technologies project.