Can Elementary Students Reason About the Invisible?
An ice cube melts when warmed, then refreezes when cooled. This simple phenomenon offers kindergarten students the opportunity to learn about solids and liquids, and the change between states. But what if young students could do more than observe macroscopic events? What if they could also develop and use models to make sense of the invisible? To find out, the Sensing Science Through Modeling Matter project developed four apps and a curriculum for kindergarten students to explain states of matter and phase change from a particulate view of matter.
Our project research focused on kindergarten students’ learning about matter. Do their concepts of matter change as they interact with the Sensing Science apps and curriculum? Are different apps associated with differences in students’ learning? Are students’ conception of particles consistent as they explain varied macroscopic phenomena?
The curriculum
The Sensing Science Through Modeling Matter curriculum includes three multiday inquiry-based lessons around modeling, states of matter, and phase change. In the first lesson, students learn about the use of models and modeling across scales. For instance, globes and maps both model the Earth. Models can also represent invisible things, such as the particles that make up matter.
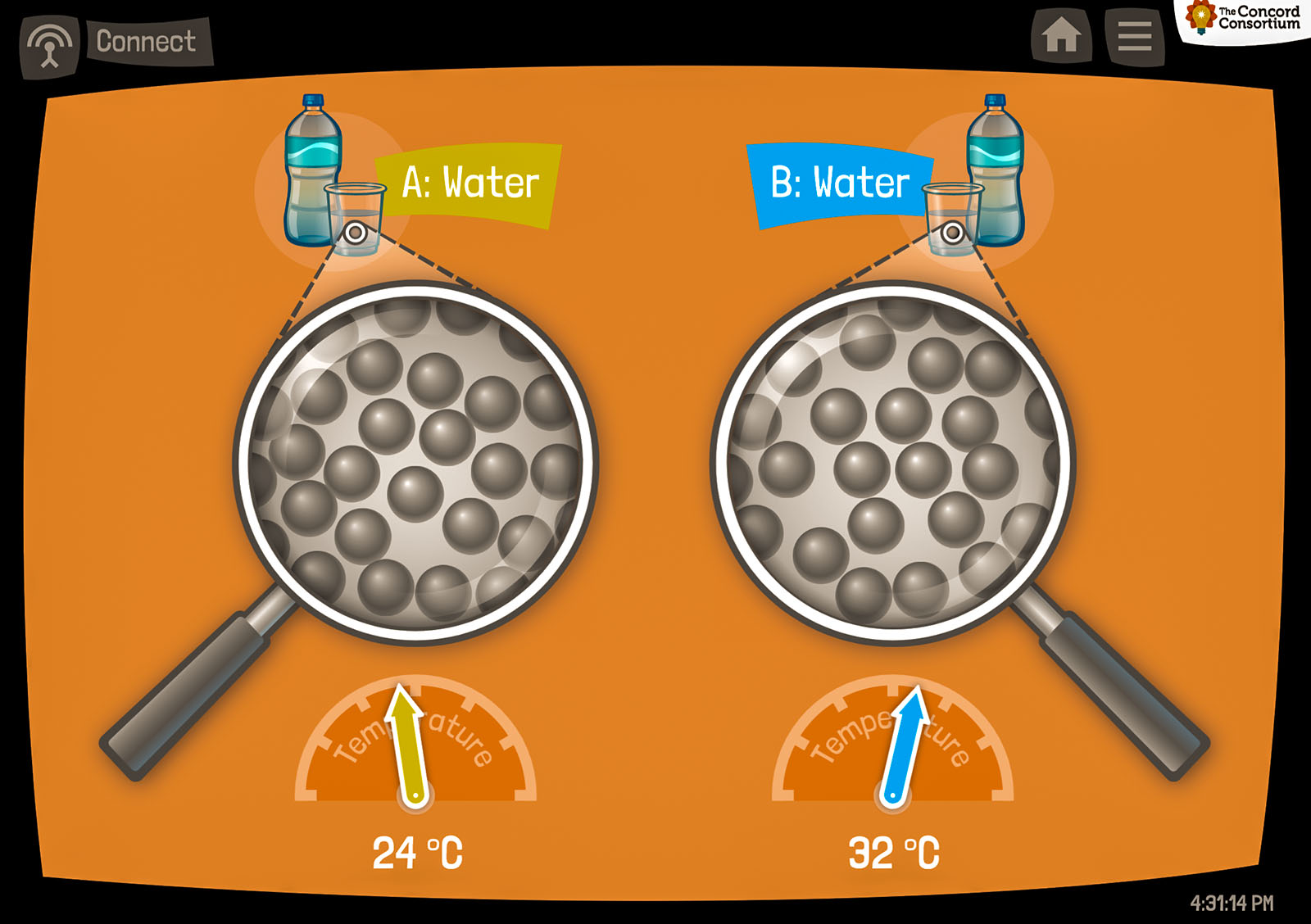
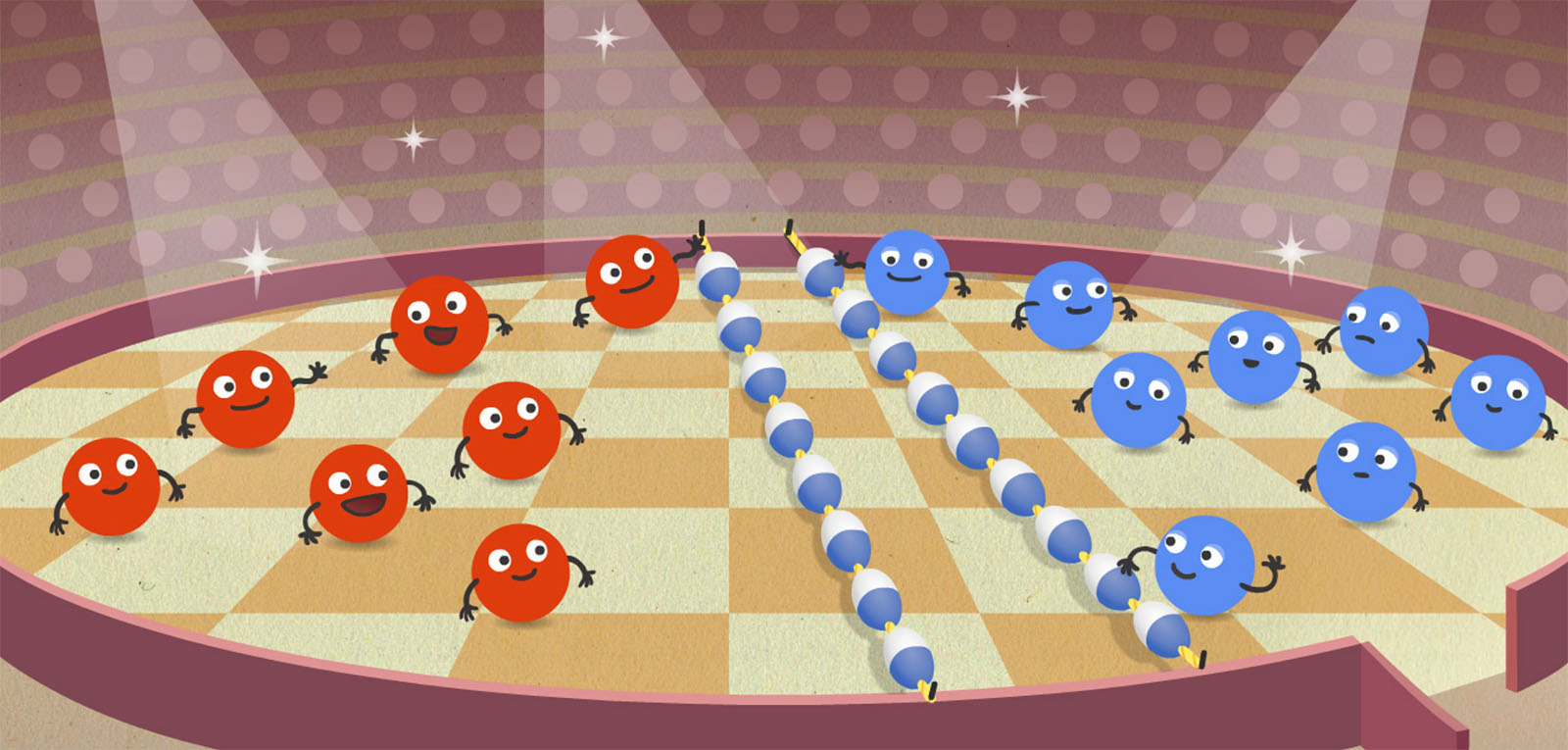
Students use an app called the Thermoscope to see inside matter; two on-screen circles act like “magnifying glasses.” When fast-acting temperature probes are placed into hot and cold water, students use the Thermoscope to observe the relationship between temperature and speed of particle movement (Figure 1). Next, students read the animated Land of Bump story, which illustrates what happens when hot and cold dancers mix together on a dance floor (Figure 2). The animated characters demonstrate a scientifically accurate computational model of water at different temperatures, and act as a metaphor for the motion of particles.
In the second lesson, students learn about states of matter and the relationship between the macroscopic properties of each state of matter and the behavior and arrangement of the microscopic particles that make up matter using one of two apps. The Particle Modeler is designed for students to discover the patterns of particles that adhere to known physical laws (Figure 3). The Thermonator allows students to set rules for the way particles move and interact, including examples that are counter to physical laws (Figure 4). Then students use their bodies to model the arrangement and motion of particles in a particular state of matter, for example, by wiggling in place or moving more freely to represent solids and liquids, respectively.


In the third lesson, students investigate melting, freezing, evaporation, and condensation. First, they observe macroscopic examples and draw their predictions of the particle model for each phase change. Next, they use the same apps to observe the microscopic particle model and revise their predicted models. Finally, they construct human models of each phase change as in the previous lesson.
Kindergarten students from seven classrooms (n = 139) in three U.S. public schools participated in the study in the spring of 2019. In Technology Group 1 (n = 95), students used the Thermoscope, the Land of Bump, and the Particle Modeler. In Technology Group 2 (n = 44), they used the Thermoscope, the Land of Bump, and the Thermonator.
Research with kindergarteners
Students worked in pairs on iPads, which allowed us to record their interactions with the apps as well as their conversations with one another through screencast recordings. Students were asked to draw and describe their understanding of the states of matter and phase change in individual project notebooks. We also interviewed students before and after they used the curriculum. Using this data, we compared changes in students’ models of matter before and after completing the curriculum.
To analyze students’ responses to interview questions we used a coding scheme based on both “top down” theoretically derived coding and “bottom up” or inductive coding based on an analysis of the data. Our coding scheme included component coding and coherence coding. The component-level coding focused on students’ responses to individual question sequences. For example, states of matter questions were coded for students’ descriptions of the a) composition of the material, b) arrangement of the material’s smallest component pieces, and c) motion of the material’s smallest component pieces.
After the component-level coding was completed, we conducted a coherence analysis to examine the degree of consistency and accuracy in students’ use of particle models across states of matter and phase change question sequences.
Results
When we analyzed pre- and post-interview data, we found significant gains in the kindergarteners’ ability to understand and use simple particle models to explain material phenomena. There were gains in component score for materiality (i.e., the ability of students to describe matter), states of matter, and phase change, as well as total score (Table 1).
| TABLE 1 | Minimum | Maximum | Mean | Standard Deviation |
|---|---|---|---|---|
| Pre Total | 12 | 150 | 43.47 | 12.93 |
| Post Total | 12 | 150 | 79.05 | 24.53 |
| Pre Materiality | 2 | 15 | 5.59 | 2.16 |
| Post Materiality | 2 | 15 | 8.43 | 2.86 |
| Pre States of Matter | 3 | 96 | 20.97 | 9.91 |
| Post States of Matter | 3 | 96 | 46.79 | 19.40 |
| Pre Phase Change | 7 | 39 | 16.92 | 4.84 |
| Post Phase Change | 7 | 39 | 23.83 | 7.44 |
Because we had designed the Particle Modeler and the Thermonator with different approaches based on different learning theories, we were also interested in the different technologies. While students in the two technology groups were equivalent in their initial knowledge of states of matter and phase changes, we found a very small but statistically significant effect on gains from pre- to post-interview total scores for technology. Students who used the Thermonator as part of Technology Group 2 had slightly higher total scores and component scores than those who used the Particle Modeler in Technology Group 1 (Table 2).
| TABLE 2 | Sample Size | Mean | Standard Deviation |
|---|---|---|---|
| Pre Total Technology Group 2 | 44 | 46.59 | 12.60 |
| Post Total Technology Group 1 | 95 | 75.92 | 25.81 |
| Post Total Technology Group 2 | 95 | 42.03 | 12.89 |
| Pre Total Technology Group 1 | 44 | 85.79 | 20.16 |
Students from all seven classrooms showed a shift towards more coherent particle model use for both states of matter and phase change phenomena. The frequency of macroscopic states of matter models decreased while the frequency of microscopic particle models increased. A similar pattern appears to hold for phase change models, although relative to the states of matter models, a greater proportion of the phase change models remained macroscopic. In particular, there appears to be a greater frequency of macroscopic or unclear models on the phase change prediction activities, suggesting that children start with less understanding of phase change phenomena than their understanding of states of matter.
Conclusion
Our study found that kindergarteners can learn to use simple particle models to explain the microscopic features and behaviors that characterize matter in solid, liquid, and gas states, and during phase changes. While this study is small and further research is needed to examine the role of different modeling practices, we believe it sheds important light on the value of introducing simple particle models to early elementary students.
Carolyn Staudt (cstaudt@concord.org) is a senior scientist.
Jamie Broadhead (jameliss.jb@gmail.com) is Head of Development and Support at Videatives, Inc.
Ala Samarapungavan (alasam@purdue.edu) is Professor of Educational Psychology at Purdue University.
Lynn A. Bryan (labryan@purdue.edu) is Professor of Science Education at Purdue University.
This material is based upon work supported by the National Science Foundation under grant DRL-1621299. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.