The Molecular Workbench (MW) software offers salient interactive simulations of electrons, atoms, and molecules that explain many phenomena from the microscopic level. What exactly is in a simulation that makes it a better teaching tool than a text book illustration?
Let’s start with a real world example. Imagine a closed glass bottle with some liquid at the bottom. There are a number of things about such a system that most of us have noticed since we were a kid. When we rotate the bottle, the liquid will always flow to fill the lowest part. When the liquid comes to rest, its surface levels off.
Now let’s heat it up. As the temperature increases, evaporation accelerates. Eventually, all liquid molecules are evaporated and the liquid vanishes–we end up with a gas that fills the entire bottle. The gas molecules are evenly distributed inside the bottle, no matter how we rotate it. Suppose the bottle is expandable. The gas molecules will fill the entire volume of it, no matter how large it becomes (the gas just gets more diluted).
These are the things we know about the difference between a gas and a liquid f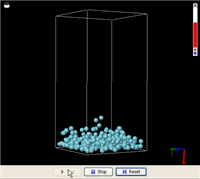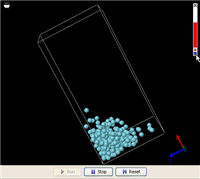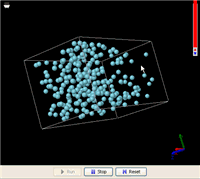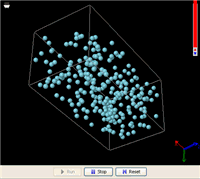 rom everyday life. Now, let’s see how a simple MW simulation can model all these facts. On the right is an animation of a liquid in a box made from an MW simulation (click this link to run it; you will need Java 5.0+). In order for the simulation to run fast enough on an ordinary computer, the liquid includes just 256 molecules. This is not a lot to be called a liquid, but it is enough to demonstrate the phenomena. In addition, a super-strong gravitational field is applied to accelerate the gravitational effect (you might have heard from someone that the gravitational effect is not important at the atomic level, but that is because the gravity on the surface of the Earth is too weak).
rom everyday life. Now, let’s see how a simple MW simulation can model all these facts. On the right is an animation of a liquid in a box made from an MW simulation (click this link to run it; you will need Java 5.0+). In order for the simulation to run fast enough on an ordinary computer, the liquid includes just 256 molecules. This is not a lot to be called a liquid, but it is enough to demonstrate the phenomena. In addition, a super-strong gravitational field is applied to accelerate the gravitational effect (you might have heard from someone that the gravitational effect is not important at the atomic level, but that is because the gravity on the surface of the Earth is too weak).
A theoretical physicist would celebrate the simulation as the triumph of theoretical physics. The fact that a computational model can describe such a variety of natural phenomena means that they have been deciphered by science.
As an ordinary user, you may not know much about what is under the hood–in fact, most of the time, you should not have to care. You may be wondering what advantages a computer simulation has, compared with just giving students a bottle of water and asking them to flip and boil it. If you are a hands-on person, you may, on the contrary, prefer giving students a bottle of water. So what is the big deal of a simulation for you?
There are a few things that the computer simulation can do for you but a bottle of water cannot. First, the simulation is literally an atomic explanation of what happens when you play with a bottle of water. The very fact that a macroscopic phenomenon can be explained with a picture of a few hundred atoms is a very important insight in science. People have probably known how water in a bottle behaves thousands of years before, but an atomic perspective was not firmly established until 100 years ago.
Second, the simulation provides an “atomic microscope” that allows users to “zoo m” into the atomic world easily because we can control it in many ways that are not realizable with a bottle of water. For example, we can navigate an “atomic camera” inside the system, or attach it to an atom. What would it look like if we could be shrunk to an atomic size and take a “space walk” or just “ride on an atom” inside a gas (the image on the right shows a screenshot in which atoms appear to run down to your face)? Kids are motivated by this kind of adventure experience, which can be supported very well by a computer simulation.
m” into the atomic world easily because we can control it in many ways that are not realizable with a bottle of water. For example, we can navigate an “atomic camera” inside the system, or attach it to an atom. What would it look like if we could be shrunk to an atomic size and take a “space walk” or just “ride on an atom” inside a gas (the image on the right shows a screenshot in which atoms appear to run down to your face)? Kids are motivated by this kind of adventure experience, which can be supported very well by a computer simulation.
Another advantage of a computer simulation is that it can be easily embedded into an electronic textbook (which recently becomes the trend due to budget crises in many states in the US). Obviously, embedding a bottle of water into an electronic textbook is much harder, if not impossible at all (I would never say that is impossible). With the support of this kind of interactive simulation, future textbooks will not be just some readable things. They will be playable delights.