Yesterday I reported evidence of tiny water permeation across a piece of paper on top of a cup of water. In order to double-check my theory, I placed a piece of transparency film on top of another cup of water and left the two cups overnight. When I came back this morning, I removed the paper and the film from the two cups and viewed the IR image of the two cups of water. To the right is the image I saw.
 The cup of water that had paper atop was cooler (the dark circle on the right) than the cup of water that had transparency film atop (the light gray circle on the left). This means evaporation was stopped by the film but not the paper. The only explanation of this effect is that water can permeate through the paper but not the film.
The cup of water that had paper atop was cooler (the dark circle on the right) than the cup of water that had transparency film atop (the light gray circle on the left). This means evaporation was stopped by the film but not the paper. The only explanation of this effect is that water can permeate through the paper but not the film.
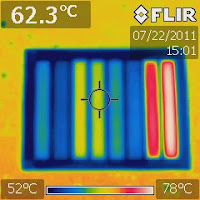
Last October, I blogged about visualizing different colors’ ability to absorb light. In that experiment, I used a table lamp as the light source. Later, I realized a flaw in that experiment because a table lamp is, after all, a point source. For the color bars to have equal illumination, we need a light source that is far far way. The sun is such a light source. So I brought the color plate outside and put it under the sun. You can now have a better idea of which color is more capable of absorbing heat. No doubt black won. To my surprise, purple and yellow have approximately the same light absorptivity. So do blue and green. Red, on the other hand, absorbed about the same amount of light as light gray. The background is white. It absorbed the least amount of light energy and appeared to be bluest in the IR image. Amazingly, paper doesn’t conduct heat well, otherwise the color bars in the IR image would not have been so well separated.