An Example of Deeply Digital Curricula: Detergents
Remember those devastating images of the burning Deepwater Horizon and satellite pictures of the huge oil slick in the Gulf of Mexico? Photos of volunteers gently bathing oil-soaked marine birds were heartrending. Those images grabbed our attention and captured the interest of science teachers and students alike. That kind of engagement is invaluable as an entry point to discovering the deeper scientific concepts of a whole suite of related ideas. The Gulf oil spill can launch students on a deep — and deeply digital — learning adventure.

Real-world connection
One prominent issue in news reports of the BP oil spill was the spectacular underwater real-time videos of detergents being pumped directly into the gushing wellhead a mile underwater. This was the first time wellhead injection had been tried and over a million gallons of detergent were used.
A class discussion of the use of detergents can raise many questions: Why add to the mess? What was BP hoping to accomplish? Did the detergents make the oil more toxic? What is a detergent anyway? Can the detergents explain why the oil slicks were less than expected, or that there were underwater plumes of oil never before observed, or that the oil may have disappeared faster than expected?
The model
To test whether a deeper understanding of detergents can be gained, without getting too technical, we created a simple atomic-scale model of oil, water, and detergents in NetLogo. The model shows a cross section of oily water with a film of tan oil above blue water (Figure 1).
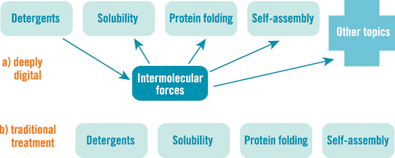
The basic ideas that govern detergents are that clusters of atoms that contain a charge “like” liquids that consist of charged molecules (such as water), and those that do not contain a charge “dislike” such liquid. The actual description should be “are in a lower energy state when surrounded by,” but “like” is easier. When something “likes water,” we say it’s hydrophilic, and if it “dislikes water,” it’s hydrophobic. And this basic idea — seen throughout biology and chemistry — explains solubility, protein folding, self-assembly, and more (Figure 2).
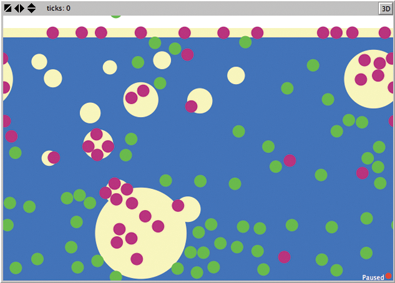


In our model, the container has been shaken to produce oil drops that float around as well as some green and magenta “mystery molecules” scattered about. As students run the model, all the oil drops coalesce and rejoin the surface, making a thicker film. Most of the green molecules end up in the water while the magenta ones end up in the oil. Students learn that the green molecules are charged and the magenta molecules are not.
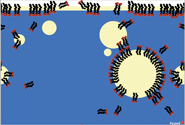
It is also possible to add another mystery molecule. As shown in Figure 3, the black molecules mostly end up at the surface between oil and water. Students are led to guess that this is because the black molecules are charged at one end and have an uncharged tail. Revealing the structure of the mystery molecules (Figure 4) shows that this is true. Indeed, the black molecules are essentially the green and magenta ones joined together, and this is precisely how detergents are constructed.
By adding more black molecules, every oil-water surface can be coated (Figure 5). Running the model shows that these coats interfere with the tendency of oil to coalesce, so these droplets last forever and, if the water is sufficiently agitated, no oil remains on the surface.
Going deeper
Deep concepts are difficult — that’s why they’re not part of traditional curricula. Usually they are relegated to advanced study and students who have mastered highly abstract, mathematical concepts. But computer models allow us to offer those same topics to beginning students in a way that is easier for them to understand. Using our detergents model, students can make informed guesses to questions about the Gulf oil spill:
Did the detergents get rid of the oil? No, they simply dispersed it.
Why was there a smaller oil slick than expected? The detergent kept some of the oil from reaching the surface.
Why was there an underwater plume? It was likely composed of detergentcoated oil droplets.
Did the detergent react with the oil? No, the dispersion works without a chemical reaction.
Did the detergent make the oil more toxic? No, the combination was no more toxic than the oil itself (which is plenty toxic); the detergent is probably only as toxic as kitchen detergents.
Is it possible that detergents contributed to eliminating the oil? The dispersed droplets have a huge surface area that might make it easier for oil-eating bacteria to digest the oil.
While this simple model does not provide definitive answers to these questions, it does enable students as informed citizens to understand the real issues at a much deeper level than most of the TV reporters, company representatives, and environmentalists did during the crisis.
Connected ideas
Educational technology has a unique capacity to make deep concepts accessible through a combination of highly interactive models, good visualizations, and well-designed learning activities. Even a qualitative understanding of deep concepts helps students make connections between topics that appear quite unconnected. And connected ideas are easier to learn, more engaging, and give a more accurate picture of the unity of science, compared to traditional approaches that treat the same phenomena separately. Our deeply digital curriculum design focuses on teaching important, deep concepts that have broad explanatory power.
A new curriculum must, and can, delve deeper into concepts. But its greatest impact would be through reordering the content sequence around these deeper ideas, not traditional subjects. We will not have fully exploited the value of technology to science teaching and learning until a totally reorganized curriculum is implemented. But that’s a topic for another time.
Robert Tinker (rtinker@concord.org) is the founder of the Concord Consortium.