Interactions and Energy: Big Ideas That Link Science Concepts
Everything in the universe is made from atoms. Virtually all phenomena we observe around us are the result of interactions between atoms and energy changes associated with those interactions: static cling is the result of attractions at the atomic level; a hurricane’s energy comes from interactions between water molecules; colds caused by viruses and bacteria are disabled when molecules made by your immune system stick to them. Much of physics, chemistry, and biology can be traced to atomic-level interactions.
The Interactions project, funded by the National Science Foundation, is developing a semester-long interdisciplinary science course based on this fundamental idea. The goal of Interactions—a collaborative partnership between the Concord Consortium, Michigan State University’s CREATE for STEM Institute, and the University of Michigan—is to support high school students in developing a deep understanding of forces and energetics involved in interactions between atoms and molecules, which can be applied to a variety of phenomena across disciplines.
Alignment to NGSS
The Framework for K-12 Science Education and the Next Generation Science Standards (NGSS) call for students to make sense of phenomena or design solutions to problems by engaging in three-dimensional learning—using scientific and engineering practices, crosscutting concepts, and disciplinary core ideas. They identify atomic-level forces and energetics as physical science disciplinary core ideas. Despite the foundational importance of these ideas, most students do not develop an understanding of them. The Interactions curriculum helps students work toward achieving many NGSS performance expectations in physical science and life science.
The curriculum especially focuses on two science and engineering practices— modeling and constructing explanations— though these practices overlap significantly with other practices, such as analyzing data and planning investigations. To facilitate these key practices, the curriculum emphasizes:
- student experience of phenomena through teacher-led demonstrations and student-run experiments
- integrating simulations that help students explore related phenomena
- conducting discourse driven by student questions and student-illustrated models that predict and explain the phenomena being studied
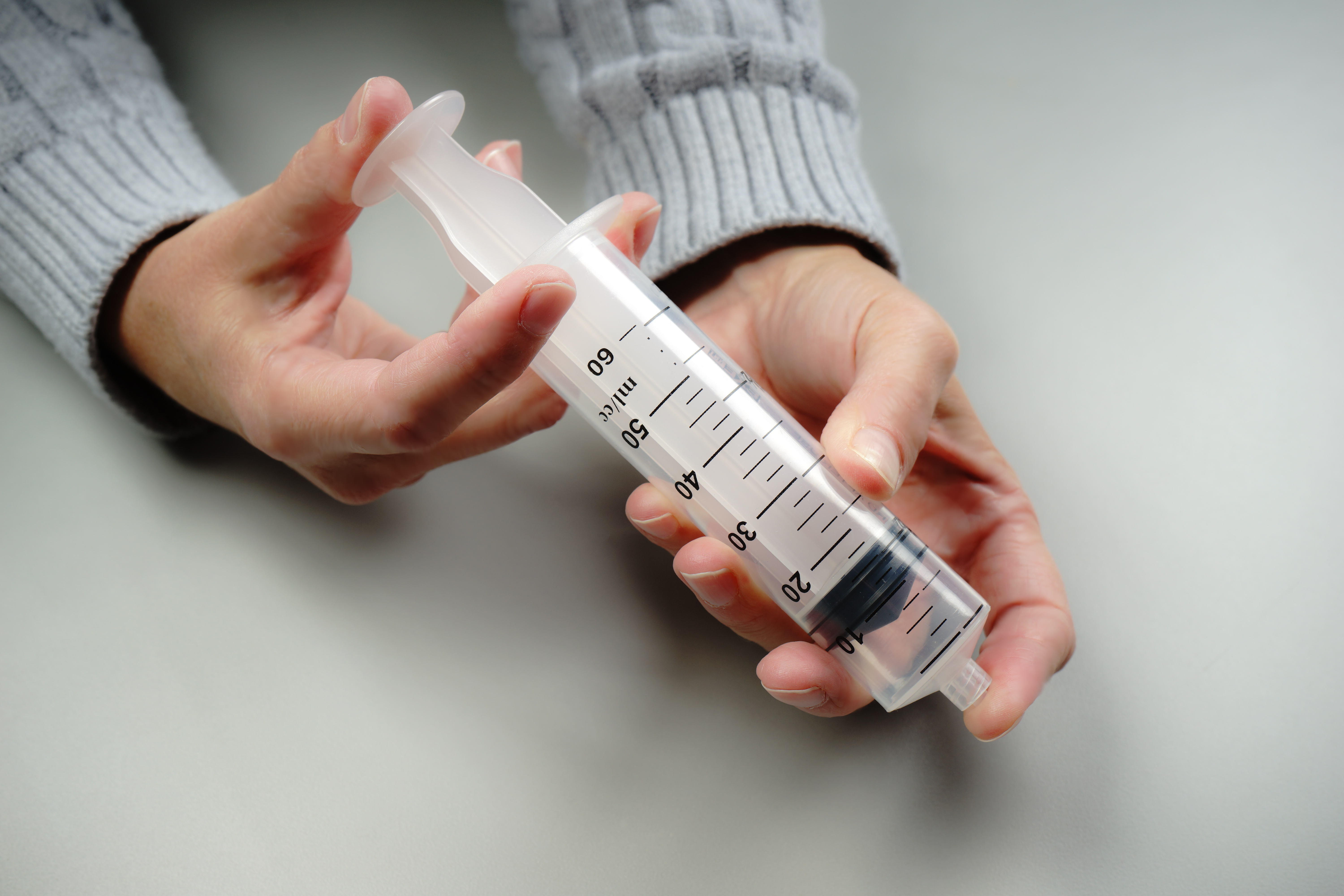
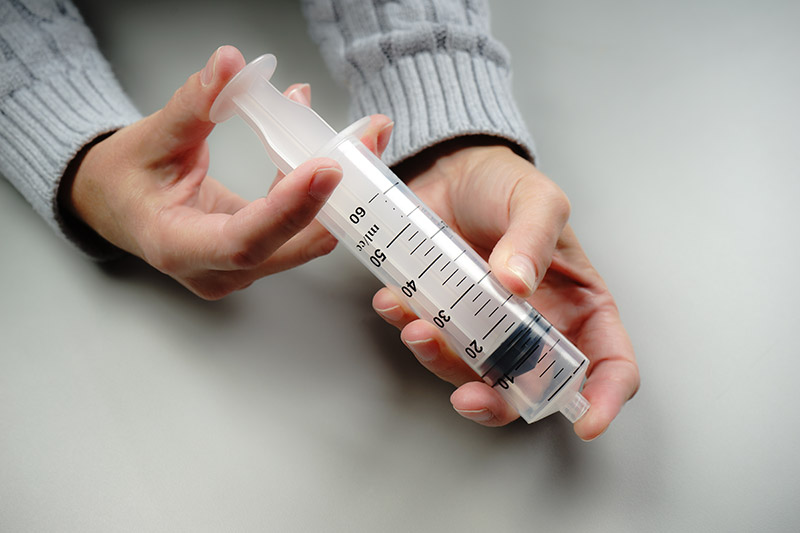
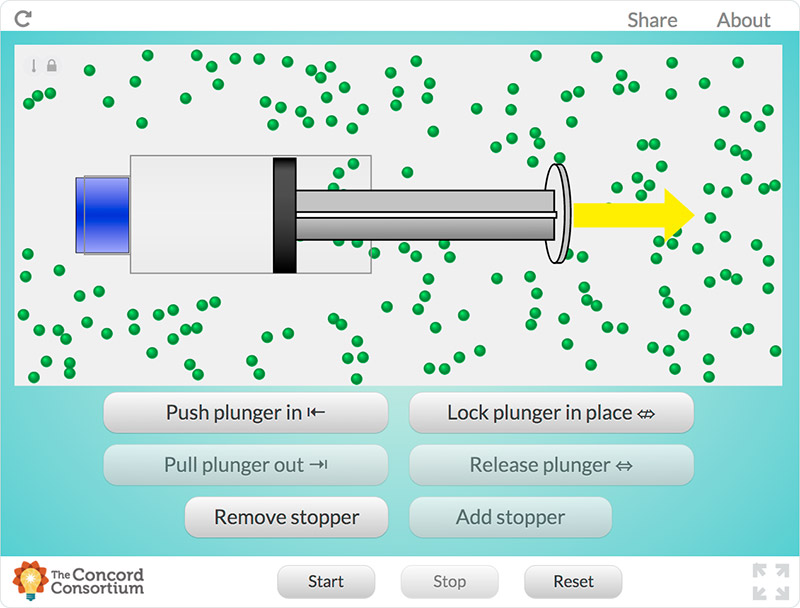
For example, students explore the particulate nature of matter in a paired set of activities. First, they experiment with a syringe: pulling the plunger out and pushing it in, sealing it and compressing the gas inside, and measuring the mass of the syringe under different conditions (Figure 1). Students then draw a model that would explain their experiences and participate in a class discussion based on their models. Next, they use a simulation of a syringe in which they can perform many of the same experiments, this time in a context in which the particulate model of matter can be tested and observed (Figure 2). Students revise their models and engage in another discussion using their work with the simulation to drive the discussion. (Examples of classroom discourse can be seen in a series of videos created by the National Science Teachers Association that highlight a classroom implementation of the Interactions curriculum.)
Curriculum overview
The disciplinary core ideas developed in the Interactions curriculum are critical for explaining many phenomena important to physics, chemistry, biology, and Earth science. Because much of what we can see at the macroscopic level is due to underlying atomic-level interactions, linking the macroscopic and microscopic worlds connects the sciences rather than putting them into narrow disciplinary boxes.
To provide motivation and context, each of the four units is guided by a driving question. Students make sense of phenomena by constructing models or writing explanations about how they occur. Physical models and computer-based simulations are used to visualize and develop an understanding of the principles that govern interactions at very small scales, so they can be used to explain phenomena at the macroscopic scale.
Unit 1 – Why do some clothes stick together when they come out of the dryer?
The unit focuses on understanding electrostatic attraction patterns at the macroscopic level, and uses the fact that neutral objects are attracted to both positively and negatively charged objects to prompt a dive into the nature of matter and the source of static charge at the atomic level.
Unit 2 – How can a small spark start a huge explosion?
Students start exploring phenomena that are best modeled using the concept of energy conservation. They are introduced to different manifestations of energy, including potential energy. As with Unit 1, the initial exploration is at the macroscopic level, and it ultimately ends in an exploration of energy at the atomic level and a basic understanding of chemical reactions.
Unit 3 – What powers a hurricane?
Students explore energy and conversion of various forms of energy that are related to intermolecular attractions and changes in state. They explore how bonds with varying polarity result in various emergent properties of substances and associated changes in energy when these molecules interact with each other.
Unit 4 – Why is a temperature of 107°F deadly?
The topic of intermolecular attractions is extended to a biological context where the structure and function of molecules are strongly linked to their shapes. Understanding why complex molecules like proteins form stable structures is approached through both intermolecular attractions (polar and non-polar) and potential energy minimization. The unit brings together models students have developed over the previous units to explain phenomena and ties together the entire curriculum.
Research
Several districts are currently field-testing the curriculum. We are researching to what depth high school students learn ideas related to interactions at the molecular level and energy changes during interactions, what types of representations and illustrative phenomena help students connect macroscopic and submicroscopic phenomena to the underlying scientific ideas that explain them, and what instructional tasks and teacher scaffolds effectively promote three-dimensional learning.
We believe that students completing this curriculum will have a solid basis for understanding and explaining much of the world around them. They’ll gain a foundation of usable knowledge that will give them a jump-start for future subject-specific courses later in high school and beyond.
Dan Damelin (ddamelin@concord.org) is a technology and curriculum developer.
Joe Krajcik (krajcik@msu.edu) directs the CREATE for STEM Institute at MSU.
This material is based upon work supported by the National Science Foundation under grant DRL-1232388. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.