Molecular Workbench and the New Standards
The Next Generation Science Standards (NGSS) raise many challenges for science teaching and learning. The standards ask teachers to cover fewer science ideas in more depth than currently while placing far greater emphasis on crosscutting concepts and science practices. Making these changes requires new content, pedagogical techniques and tools. Powerful computer-based tools like Molecular Workbench can address these challenges by allowing students to learn from their own investigations how a simple set of core concepts can explain a wide range of observations that cut across fields and disciplines. This can build student understanding of science practices, crosscutting themes and core ideas all at the same time.
Molecular Workbench
Funded by the National Science Foundation and Google.org, Molecular Workbench is a powerful and versatile cluster of opensource resources for modeling atomic-scale interactions and exploring their macroscopic effects. Every Molecular Workbench model is based on a general physics engine that calculates the forces acting at the atomic level, adds rules for photons, chemical bonds and macromolecules, and applies Newton’s laws to determine the resulting motion. When the interactions of hundreds of atoms are computed this way, collective behavior emerges that can illustrate macroscopic effects as varied as phase change, diffusion and explosions.
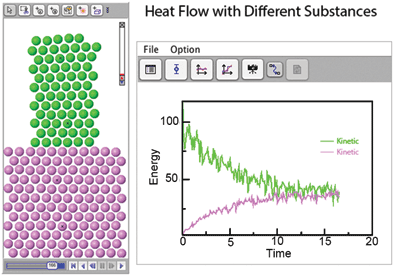
The Molecular Workbench is also a model maker that can create hundreds of different models. Having students use this capacity to modify models or make their own is a great way to learn. For example, a teacher could challenge students to explore the effect of atomic mass on thermal conductivity (Figure 1). Or, using the online user manual, students can make simple models themselves, the best way to understand the underlying science.
But most learning activities need more than just a model; they require background information, instructions and assessment items, all of which can be created with the activity maker. Figure 2 shows pages from a biotechnology activity and an activity on spectroscopy, which illustrate how Molecular Workbench provides these authoring capabilities.
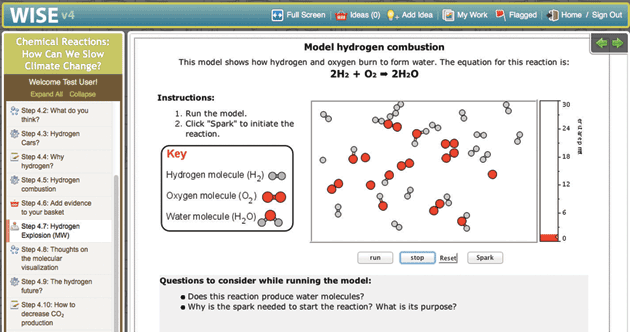
Molecular Workbench is also a component maker. It can be converted into applets and scripts that are easy to incorporate into other platforms and run entirely in Web browsers. Many of our models are used in the University of California, Berkeley’s WISE projects (Figure 3). The browser-based Next-Generation Molecular Workbench extends MW’s flexibility to more mobile devices and simplifies integrations even further (Figure 4). Easy to embed and share, these JavaScript models are being used by MIT in an edX Massive Open Online Course, embedded in Data Games, a data exploration tool from KCP Technologies, and included in online textbooks and supplements.

Scientific practices
By running models and observing many runs under different conditions, students gain important insights into the underlying processes. Figure 1 shows a simple model that can be used to explore factors affecting heat flow between different substances. Students can observe the transfer of energy caused by interactions between atoms and see a real-time graph of the temperature. Careful study of the model can expose details such as thermal equilibrium and the relative contributions of mass and velocity in the kinetic energy of individual atoms.
This model, as with most Molecular Workbench models, is suitable for a wide variety of applications and grade levels. Younger students may gain great value from simply visualizing how temperature relates to molecular motion and spreads. More advanced students may learn important lessons about equilibrium while higher education students can explore proportionality between temperature and kinetic energy.
Explorations like these based on Molecular Workbench models can be structured to include all eight NGSS scientific practices so students can experience how science is done. And the visual nature of these models helps ensure that students at a disadvantage in language or math skills are not left behind.
Crosscutting concepts
The NGSS also identify seven crosscutting concepts that represent important core ideas encountered across science and engineering. Students need to learn these concepts in context rather than in isolation. Molecular Workbench is ideal for understanding them in ways that further deep learning.
Figure 2 (inset) shows a Molecular Workbench activity containing a model of hybridization, a technique frequently used in biotechnology. Students can observe that random motion breaks up any pairing of the four-group molecules unless they have complementary charges. Experimenting with this model shows vividly that at too low a temperature all molecules pair at random, while at too hot a temperature none pair. This brief interaction demonstrates three crosscutting concepts at the same time: it highlights a cause and effect interaction critically entwined with the inherent stability of a system at the heart of a biochemical process relating structure and function.
Because of the variety of physical models within Molecular Workbench, teachers can easily find many examples in which crosscutting ideas can be applied to appropriate STEM concepts. A teacher might choose to focus on models with very different content, all of which can be understood by applying energy conservation. The models in Figure 2 and 3 illustrate this idea.
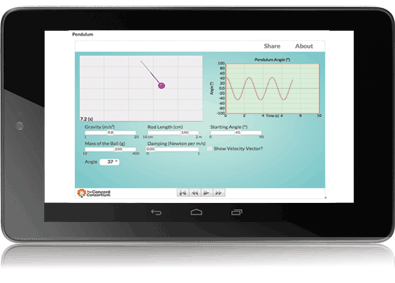
Figure 2 also shows an activity in which atoms can have quantum states of various energies (when an atom is in an excited state, a dashed circle appears around the atom). Students discover that these excited states can be caused either by collisions between atoms or by photons (short, wiggly lines). Once excited, the atoms can transfer their excitation energy—in quantified packets—either through collisions or by emitting a photon. Thus, energy is converted between kinetic energy, excitation energy and light energy, and is conserved in each instance. These ideas can be applied to a wide range of topics, including spectra, fluorescence, lasers and LEDs.
The atoms in Figure 3 can make and break chemical bonds. Since it takes energy to break a bond and that same energy is released when a bond is made, energy is again conserved, but converted between types of energy. In this case, the conversion occurs between chemical and kinetic energy. In the figure showing the reaction that produces water from H2 and O2, this simple idea is applied to explosions. The mixture of these two gases is stable because a bond must be broken before H2O can be produced, but a tiny spark that breaks only one bond can initiate a chain reaction, rapidly consuming all the oxygen and hydrogen—the atomic view of an explosion. Starting with these models the crosscutting concept of energy conservation can be applied to a wide range of chemical phenomena: catalysis, polymerization, net energy of a reaction, reaction pathways and partial reactions.
Conclusion
The strength of Molecular Workbench is the engine that computes the evolution of a few basic laws into complex systems. The degree to which Molecular Workbench can support large portions of the Next Generation Science Standards suggests that a small collection of such powerful modeling tools could revolutionize science instruction. Someday, a few computational models similar in power and flexibility to Molecular Workbench will address topics such as heat transport, evolution, geophysics, astronomy, engineering design, quantum mechanics and fluids to support learning across the entirety of the Next Generation Science Standards.
Robert Tinker (rtinker@concord.org) is President Emeritus of the Concord Consortium.
Chad Dorsey (cdorsey@concord.org) is President of the Concord Consortium.