Monday’s Lesson: Digital Inquiry in the One-Computer Classroom
The Rhode Island Technology Experiences for Students and Teachers (RI-ITEST) project contains some of the best deeply digital resources for doing inquiry in the high school science classroom. What exactly does it mean to “do inquiry” with digital resources? And what does it look like, especially when you have just one computer?
The RI-ITEST activities were created by the Science of Atoms and Molecules project, which developed 24 short instructional units in physics, chemistry, and biology that were built around interactive computer models with embedded assessments. Though these activities were originally designed for an individual or pair of students, there are several reasons why a teacher may want to use them in a one-computer mode with the whole class. Numbers of computers, reliability of available computers, or the timing of when computers are available may limit student computer access.
Even when technology access is not an issue, best practices call for bringing the class together for discussion after students work with models on their own. But doing whole class inquiry can be difficult, especially with interactive models displayed from one computer at the front of the class.
To facilitate inquiry in a whole class mode, try these strategies:
Brainstorm. Start with questions that allow every student to contribute (e.g., ask students to simply describe what they see). Since most students will be able to contribute, they will be more likely to chime in to later discussions.
Have students manipulate models. Ask the whole class what to do with a model. Try taking a vote (e.g., “Should we increase the concentration or decrease it?”) or calling on individual students to try out their ideas by inviting them to the front of the class to set up the model.
Use models to back up claims. When students make a claim, have them set up and interpret the model. Invite discussion and have students come up with counter examples (or present a counter claim yourself, if necessary).
Now, let’s see how this works in the “Diffusion, Osmosis, and Active Transport” activity (ri-itest.portal.concord.org/ diy/view/68/type/ExternalOtrunkActivity/).
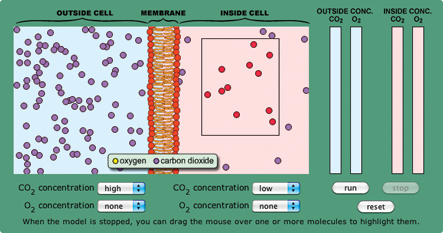
- Set up the model on page 2 of the activity with a high CO2 concentration on one side of the cell membrane and low CO2 concentration on the other. Run it and ask students what they see. If they say CO2 is going from the high to low concentration area, ask them to demonstrate this in multiple ways.
- Reset the model and highlight the molecules in the low concentration area by dragging the mouse over them (Figure 1). When you run the model again, students should see some molecules move from the low to high concentration area. Ask: How is equilibrium reached if molecules are moving both ways?
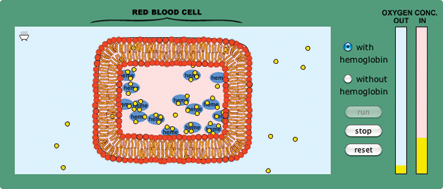
- Since most students think that equilibrium is defined by equal concentrations on both sides, challenge their notion of equilibrium by running the model on page 5 of the activity with and without hemoglobin. Ask students to debate whether the “with hemoglobin” option reaches equilibrium (Figure 2 ). The hemoglobin causes a new equilibrium with a higher concentration of oxygen inside the cell. This highlights the fact that equilibrium means equal rates of exchange into and out of the cell, not that there are equal concentrations.
Try it out
Most interactive models can be adapted for the one-computer classroom and used as an effective means for engaging students in scientific inquiry. Give it a try! Some of my best classes have been done this way.
Dan Damelin (ddamelin@concord.org) directs the RI-ITEST project.
This material is based upon work supported by the National Science Foundation under grant DRL-0737649. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.