Monday’s Lesson: Teaching About Molecules with Multiple Representations
Thousands of different chemical reactions are taking place in your body as you read this sentence. Ions and molecules are colliding, diffusing, and reacting at a frenetic pace. In other words, molecules and their interactions are the very stuff of biology and scientists are learning more about these biomolecular reactions every day. Their research fuels the biotechnology industry and guides the search for a cure for cancer and many other diseases. Because of its importance in modern research and industry, biochemistry is now taught in biology courses, including high school biology.
Teaching chemistry in biology
As content leans more toward the molecular level and an understanding of biochemical processes, biology teachers are challenged to teach chemistry to students who frequently do not have a significant grounding in chemistry. Additionally, the types of molecules biology teachers refer to are often large and complex. To make these molecules more understandable, teachers use different kinds of representations: Fischer projections, abbreviated names for large molecules like NADH, and cartoon views for really large molecules like proteins.
Most students enter biology only having experienced simple formulas and ball and stick views of basic molecules. When faced with unfamiliar molecular representations, students may feel as if their previous knowledge about molecules doesn’t apply in biology class. One way to help students understand the chemistry happening in their biology class is to present molecules in various representations at the same time. In our “Cellular Respiration” activity, developed by the Science of Atoms and Molecules project, we do just that.
Glycolysis and the Krebs cycle
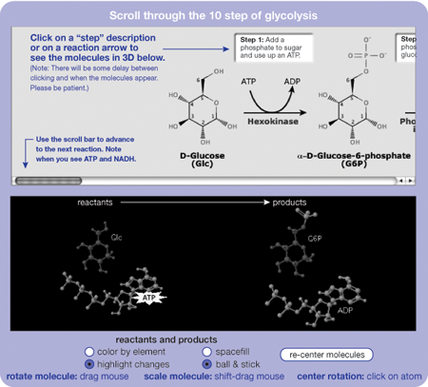
The Cellular Respiration activity begins by teaching about glycolysis and the Krebs cycle. Typically this is taught using only the molecular representations shown at the top of the figure at left.
 Students can click on any of the reaction arrows to see the molecules represented in the more familiar ball and stick form. The interactive 3D molecules also give students different ways to view the molecules, for example, by coloring them to highlight what has happened in the reaction. Students can then follow how the atoms are rearranged to form new molecules.
Students can click on any of the reaction arrows to see the molecules represented in the more familiar ball and stick form. The interactive 3D molecules also give students different ways to view the molecules, for example, by coloring them to highlight what has happened in the reaction. Students can then follow how the atoms are rearranged to form new molecules.
The goal is not for students to memorize every step of glycolysis or the Krebs cycle, but to provide tools to help students make connections between different representations of molecular structures (e.g., 3D, ball and stick, and formulas) and to help them see patterns in how atoms are exchanged during reactions by giving them control over various ways of looking at the molecules.
Biochemistry and the misrepresentation of dynamic processes
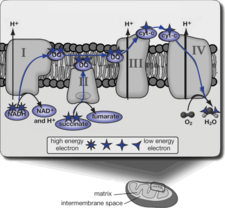
The atomic world is frenetic and random. Molecules vibrate, move, collide, and react at a phenomenal pace. They don’t know where they are or where they are going, yet biochemical pathways are commonly diagrammed as if things happen in an ordered sequence and in a directed manner.
The electron transport chain involves many protein complexes and cofactors, some of which are anchored to the inner mitochondrial membrane, and some of which move, floating in random directions, buffeted by water and other molecules surrounding them. But that dynamic unchoreographed action is not evident in the typical illustrated diagram (see figure above). Here it appears as if NADH and succinate “know” just what to do and that the path of the electrons follows a smooth unbroken sequence from beginning to end. While these diagrams are very helpful in understanding the overall outcome, they oversimplify the true nature of the dynamic world of atoms and molecules.
In the latter parts of the Cellular Respiration activity the models depict a more realistic view of what is happening in the electron transport chain (see figure below). The chemical energy (represented as high energy electrons) produced in molecules generated by glycolysis and the Krebs cycle is used to push hydrogen ions from one side of the inner mitochondrial membrane to the other. Eventually the energy stored in the high hydrogen ion concentration is used to make ATP, a crucial molecule that powers many chemical processes in your body.
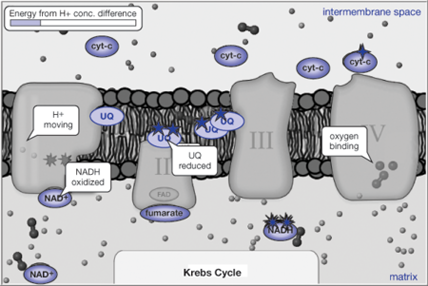
Viewing a dynamic model of such a complex system can be overwhelming at first, but it can provide insights that static pictures just can’t convey. To begin, focus your attention on one protein complex at a time and study it until you get a sense of its function isolated from the rest of the electron transport chain. (The model facilitates this: when you click on one of the protein complexes, only that complex will be visible.)
Expanding our view of the biomolecular world
By helping students to make connections through multiple representations—from a molecular formula to a complex illustration to a dynamic model—we give them tools to better understand the world around them. Experts easily translate from one representation to another and know which one is best for the type of critical thinking they need to do at that moment. Our goal is to help students learn to think more like experts, to facilitate their increased understanding of the molecular world, and to provide them with the tools experts use as well as the facility to know when to use them.