Monday’s Lesson: Exploring Atomic Structure
The Interactions project is developing a new interdisciplinary semester-long course that lays the foundation for deeper understanding in physics, chemistry and biology. This ninth grade course, developed in partnership with the CREATE for STEM Institute at Michigan State University and the University of Michigan, is inspired by the Framework for K-12 Science Education and the NGSS, which encourage students to learn science by engaging in science practices.
The curriculum is based on the idea that much of what we see around us is the result of interactions between atoms and molecules. Many phenomena across multiple fields can be explained using this framework. At the atomic level, the primary interaction is based on electrostatic fields and forces. To understand why, students first need to understand the composition of atoms.
Atomic theory is one of the most challenging topics to address in the constructivist classroom. Our Next-Generation Molecular Workbench interactives are designed to help. They reveal the emergent behavior of atoms and molecules and give students the opportunity to use data from observations to reason about atoms and their underlying structure.
Try these interactives
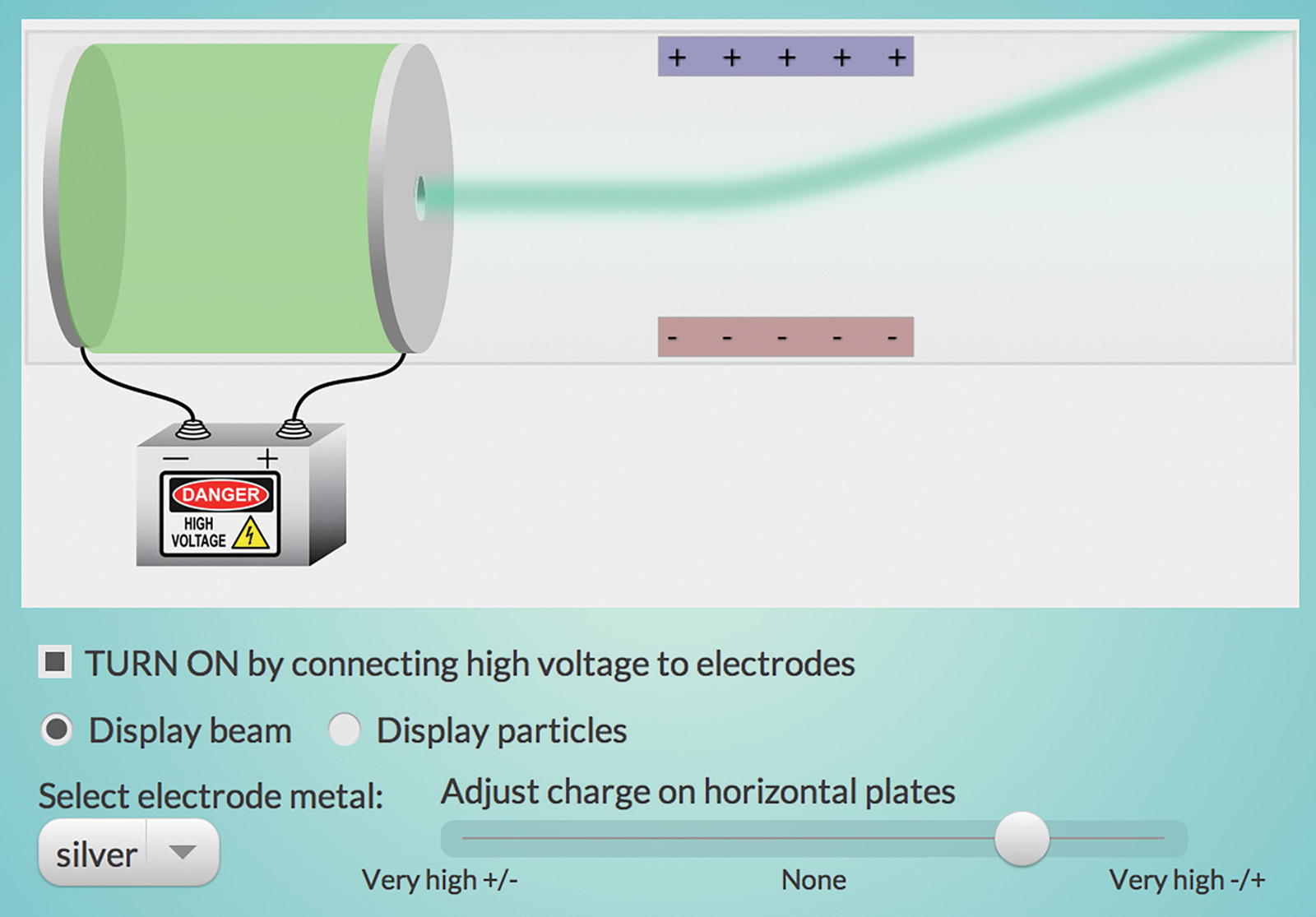
The “Thomson Experiment” interactive (Figure 1) simulates one of J. J. Thomson’s experiments with cathode rays that led to the discovery of the first subatomic particle—the electron.
Apply a high voltage across electrodes made of various metals to produce “cathode rays.” The hole in one of the electrodes allows cathode rays to pass through the plate. Adjust the charge on the plates and select different metal electrodes. Did you find that the particles always behave the same way when deflected by the charged plates, no matter which metal was used in the electrode?
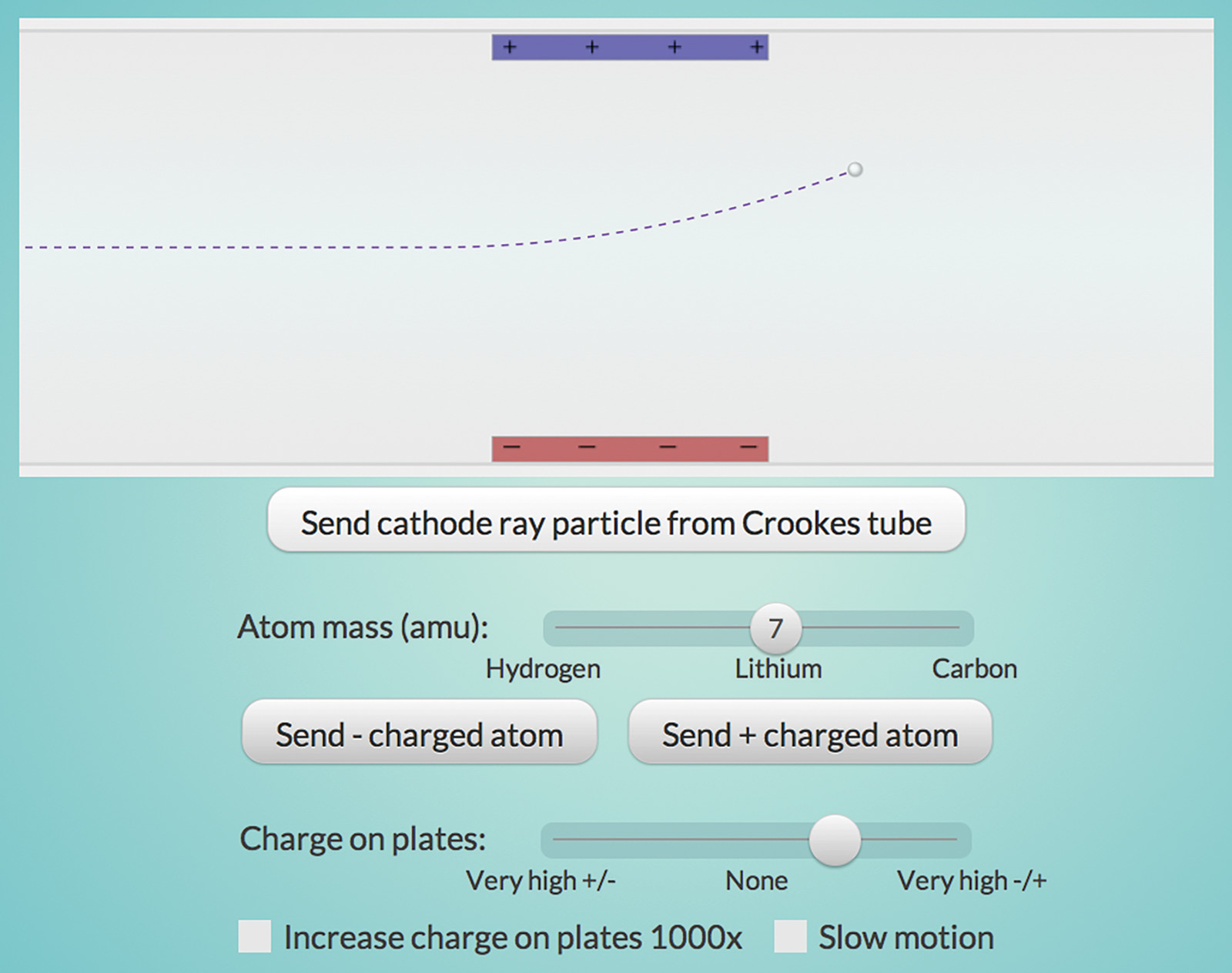
Now, compare the behavior of the negatively charged “cathode ray particles” with that of atoms in the “Electron Properties” interactive (Figure 2). Cathode ray particles have much less mass than even hydrogen, the lowest mass atom. Combined with the fact that all metals seemed to produce the same particles, Thomson concluded that cathode ray particles must be part of all atoms. The first subatomic particle to be discovered was called the electron.
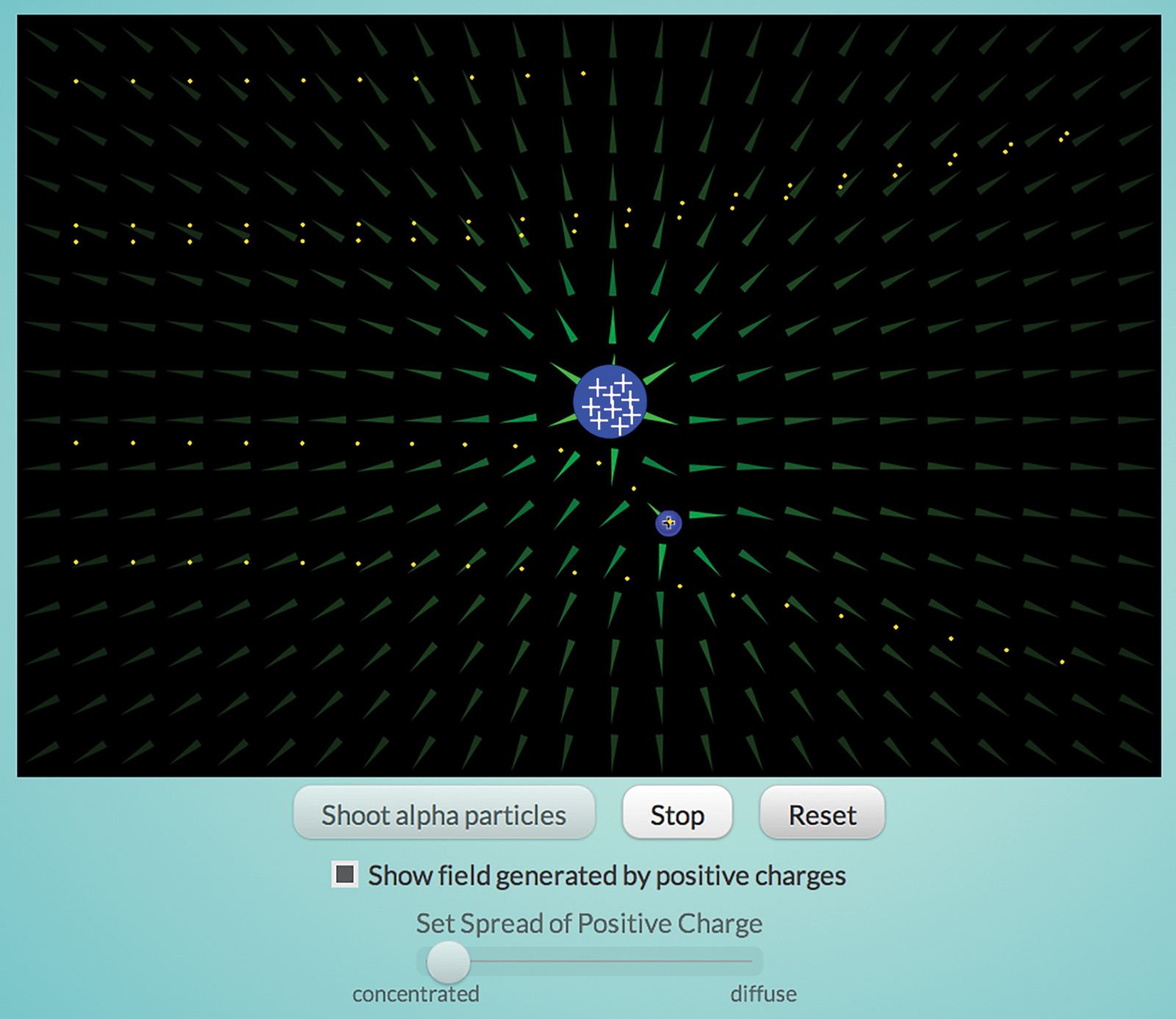
Once negative electrons were found, there had to be a positive component of the atom as well. Otherwise, everything would be negatively charged and repel everything else. One possible model, commonly called the Plum Pudding model, postulated that the positive charge was dispersed throughout the atom with the negative electrons floating around in it.
The “Rutherford” interactive (Figure 3) simulates Ernest Rutherford’s experiment to test this model. He shot alpha particles—very high-energy, high-speed, positively charged helium atoms—at a thin piece of gold foil. If the positive part of atoms was dispersed, he reasoned, the electric field generated by those atoms would not be strong enough to deflect the alpha particles. However, some particles were deflected and some bounced back from the foil. Only a concentrated charge could generate a strong enough field to do this, thus Rutherford concluded that atoms have a tiny, dense, positively charged nucleus.
These interactives are part of the student investigation: “How can we learn about something we can’t see?“
Dan Damelin (ddamelin@concord.org) is a technology and curriculum developer.
This material is based upon work supported by the National Science Foundation under grant DRL-1232388. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.